At-Home Covid-19 Tests Are Here, But Can You Trust Them?
Updated: Mar. 30, 2022
The FDA has approved some at-home viral tests to detect the coronavirus that causes Covid-19. Here's what to know about accuracy and safety. (Watch out for fakes.)
Testing for Covid-19 has become somewhat easier for people in the United States. While there was a severe shortage of tests in the early weeks and months of the pandemic, more recently, many states have made testing sites available for people who have exhibited symptoms of the disease and even those who are asymptomatic.
According to the Centers for Disease Control and Prevention (CDC), as of June 4, more than 19.8 million tests have been performed in the U.S. (About 11% were positive.) Depending on how states report testing, these figures include two types of tests—those that check to see if you currently have an infection with SARS-CoV-2, the virus that causes Covid-19, and antibody tests, which can determine if you have been infected with the virus in the past.
The tests used to diagnose a current infection rely on a nasopharyngeal (back of the throat and nose) swab and look for the genetic material of the virus. These types of tests, known as viral tests, can pick up the virus while it’s actively in your body. Once the virus is gone, it can take 1 to 3 weeks for the body to generate antibodies. A blood test can then look for the antibodies to see if you were infected with the virus at some point in the past.
Both types of tests are available through testing facilities, hospitals, and doctor’s offices. However, some commercial medical manufacturers such as Everlywell and Pixel by LabCorp are also selling at-home collection kits that use the nasopharyngeal swab to detect an active infection. The U.S. Food and Drug Administration (FDA) has also approved an at-home collection kit that uses saliva, which was developed by Rutgers Clinical Genomics Laboratory. (There are no approved, at-home tests for antibodies yet.)
In general, tests have been authorized by the FDA under an emergency use authorization (EUA), meaning they aren’t officially approved in the traditional sense but have been authorized for use because of the public health crisis. The newest rapid Covid-19 antigen test approved by the FDA provides results in 15 minutes and will cost just $5
But not all Covid-19 at-home tests are the same. Early in the pandemic on March 20, the FDA issued a consumer alert about Covid-19 tests, warning that some may be “fraudulent” and “unauthorized.”
Although now that there are some authorized home tests available to consumers, how reliable will they be and can they help fight the current pandemic?
There are different kinds of at-home tests
In general, home tests allow you to test for—or monitor—diseases or conditions at home. They offer convenience and privacy and can be cost effective, says the FDA.
These tests fall into one of two categories, says Jacqueline Linnes, PhD, Marta E. Gross assistant professor of biomedical engineering at Purdue University in West Lafayette, Indiana. The first type includes at-home collection tests that involve taking a sample at home (for example, a nasal swab or saliva sample) then sending it to a lab for actual testing and results. The second type of at-home test allows you to both take the sample and get the result at home.
There are dozens of both types on the market, from home pregnancy kits to tests for HIV and other sexually transmitted diseases, hepatitis, urinary tract infections, blood glucose levels and even ketosis tests for people following the keto diet, says Dr. Linnes, whose lab is working on a handheld portable device that could test for Covid-19 and other viruses.
Many types of at-home tests are accurate
The reliability of a test really depends on which test you’re talking about. Home tests that have been approved by the FDA and have been around a while are generally quite reliable. (Look for their accuracy rating on the packaging or the manufacturer’s website.) One home HIV test, says Dr. Linnes, is 99.9 percent accurate.
“One of the keys to a reliable home test will be adequate sample collection,” says S. Wesley Long, MD, assistant professor of pathology and genomic medicine at Houston Methodist Hospital. “Home testing or monitoring for conditions like hypertension or diabetes is well established and useful, and typically uses FDA-approved medical devices.” Dr. Long points out, however, that this type of test is for monitoring people who have already been diagnosed with a condition. The tests are not used to actually diagnose the condition. Home testing for diagnosis is relatively new.
Another issue is the quality of the instructions. “I think that people can do these things if you give them really good instructions,” says Dr. Linnes.
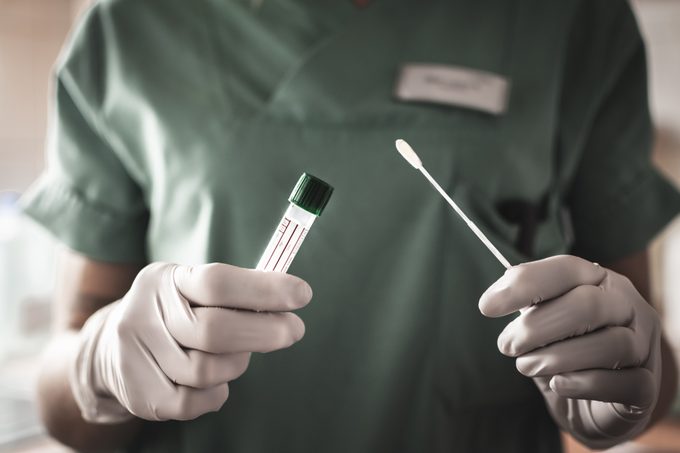
At-home Covid-19 tests have pluses and minuses
Both the Everlywell and Pixel tests are at-home collection tests. This means you get a kit that contains everything you need to get a nasopharyngeal swab. You are responsible for doing the actual swab and sending it off to the lab. The lab will do the testing and get the results, often within 48 hours.
The collection method is a concern for some experts. On the podcast, Sawbones: A Marital Tour of Misguided Medicine, Sydnee McElroy, MD, said the main problem was the consumer’s ability—or inability—to collect an adequate sample on their own. “It’s not something you as a lay consumer at home would necessarily be skilled at doing,” she said in the podcast. “It’s difficult for me to swab myself and I know how to do this. I’ve been trained.” That would throw into doubt the validity of any results. “If you don’t get a decent sample because it is so uncomfortable then you’re going to get a false negative result and then you might think you don’t have it when you do, which is bad.”
A false negative result, says Dr. Long, “could lead to individuals who have Covid-19 disease thinking they are ‘safe’ and then moving into the community and potentially infecting others, continuing the spread of this disease.” Dr. Long adds that it may be difficult to gauge reliability of at-home Covid-19 tests if a lot of products are coming onto the market at the same time.
According to Everlywell’s website, the in-home test costs $109 for consumers, although a company spokesperson says that amount only covers the costs of what it takes to make the test. The company sends the samples that people collect to labs that have been certified by the government.
The sensitivity and specificity ranges to date for the testing exceed 97% and 93% respectively, the spokesperson said in a March 26 email to The Healthy. (Sensitivity is the ability to pick up cases and avoid false negatives—situations where someone has the condition but tests negative. Specificity is the ability to diagnose Covid-19 accurately and avoid false positives—situations where a test says someone has the condition when they actually do not.)
The company also plans to provide free telehealth consults with an independent physician for people who test positive.”They’re not expecting the user to interpret the results,” says Dr. Linnes.
Pixel by LabCorp is not charging any upfront costs for its Covid-19 test. It will either seek payment from insurance or from the government if the person doesn’t have insurance. You have to complete a survey on the company’s website to see if you’re eligible for the test, then they’ll send out your collection kit and file insurance information for you.
Another test from the University of Washington involves patients collecting their own sample, but only in a healthcare setting. This helps protect workers, saves precious protective equipment, and is fast, the University says. They conducted a study, in partnership with the Bill & Melinda Gates Foundation and Quest Diagnostics, and found that a self-administered sample collection in a healthcare setting picked up 90% of coronavirus-positive patients.
Beware of scams
“I would only trust a home test that has received EUA (Emergency Use Authorization) clearance from the FDA or is FDA approved, and only if the instructions on sample collection were very detailed and explicit,” says Dr. Long. “People need to understand how critical proper sample collection is to diagnosing Covid-19.” You can find the products and tests that have been given an EUA authorization on the FDA’s website.
Dr. Long points to a New England Journal of Medicine video which demonstrates collecting a sample. “It can be fairly easy to collect an (nasopharyngeal) specimen from yourself, once you have the hang of it,” he says.
A test may not be necessary if you have relatively mild symptoms. “If you are stable enough to stay home, you don’t necessarily need to know you have Covid-19 because it will not change your management,” says Dr. Long. Even if you’re sick and have symptoms like a mild fever and cough, you should be staying home, he adds.
And if you’re not ill, the advice is still to stay at home.
What about testing after you’ve been sick?
Right now the priority groups for viral tests are still people with symptoms who are already in the hospital, are healthcare facility workers, or in long-term care settings, followed by people with symptoms who are not yet hospitalized or those who don’t have symptoms but are a priority to health departments or doctors for any reason, says the CDC.
At-home viral tests won’t tell you if you’ve been infected in the past—only if you have the infection at the time the sample is collected. A nose or throat swab won’t pick up on the antibodies found in blood that can indicate a past infection.
On April 1, the FDA approved the first diagnostic test to look for coronavirus antibodies in blood, which is made by the company Cellex Inc. There are now many other antibody tests and you can see a doctor or healthcare provider to get an antibody test, according to the CDC. Antibody tests generally should not be used to diagnose Covid-19, and they aren’t at-home tests.
“After infection, [blood] tests can look for antibodies which have developed in your body to fight the infection to tell you if you’ve been infected previously or not.” These antibodies “are being studied both for their long-term protective effects as well as potential therapeutic use,” he adds. (For more information, read about how plasma from Covid-19 survivors may be a lifesaving treatment.)
Do you have a story to share about coronavirus? Click this link to share your Covid-19 story with us.
