Coronavirus Testing: Everything You Need to Know
Updated: Mar. 30, 2022
There are tons of tests for Covid-19 out there and more coming every day. Learn about the different types and how they can help
Testing for Covid-19 is one of the most important issues of the pandemic, both to stem the spread of the virus and to gauge when and how to reopen society.
There are an almost dizzying number of tests out there, with more arriving seemingly every day. Despite, this, there’s still a shortage, and the need is not going away.
“If we had had more testing earlier, it would have helped, but there is going to be an ongoing need for testing especially as we start to see a decreasing number of cases and we start to consider reopening society again,” says Adam Seegmiller, MD, PhD, professor and vice-chair of pathology, microbiology, and immunology at Vanderbilt University School of Medicine in Nashville.
 And “knowing who is infected and should self-isolate helps us flatten the curve by preventing ongoing transmission from sick to healthy people,” adds S. Wesley Long, MD, PhD, assistant professor of pathology and genomic medicine at Houston Methodist Hospital.
And “knowing who is infected and should self-isolate helps us flatten the curve by preventing ongoing transmission from sick to healthy people,” adds S. Wesley Long, MD, PhD, assistant professor of pathology and genomic medicine at Houston Methodist Hospital.
The landscape of Covid-19 testing is complicated, but it’s important to understand, so you can protect yourself and the people you care about. There are two basic types of tests for Covid-19. One confirms the presence of the virus itself and the other checks for antibodies, proteins your body makes that indicate that you’ve had the virus. Beyond that, there are a lot of variables. Here are the basics:
Testing for Covid-19 infection
These are tests that determine whether someone has actually been infected with Covid-19, as opposed to no infection or infection with something else.
“Testing for the virus itself is critical for people who are seriously ill to know what’s causing the illness and how to treat it,” says Dr. Seegmiller, who is also executive medical director of clinical pathology at Vanderbilt. This test is also important for people who aren’t seriously ill so they can take precautions (like quarantining) to prevent spreading the virus, as well as for healthcare providers so they can protect themselves when working with a patient, he adds.
All of these tests look for the presence of Covid-19 RNA, part of the organism’s genetic make-up. “The virus has a very specific genetic sequence,” explains Rodney Ho, PhD, professor of pharmaceutics and bioengineering at the University of Washington in Seattle.
Here are nine symptoms of Covid-19 to watch for.
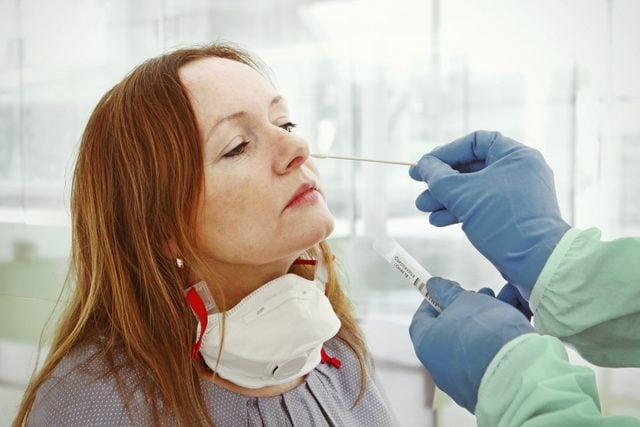
How the viral tests differ
Typically tests to look for the actual presence of Covid-19 virus use samples from your respiratory system says Jonathan Schmitz, MD, PhD, assistant professor of pathology, microbiology, and immunology at Vanderbilt University School of Medicine.
But these specimens can be collected from different places in your body. Nasopharyngeal swabs (back of the nose and throat), oropharyngeal (throat), nostrils, and saliva all can work, says Dr. Schmitz.
Tests also differ in how the samples are collected (by a healthcare worker or by the patients themselves), by how long it takes to get results (sometimes as quickly as 10-15 minutes at the bedside), and by who makes them—universities, governmental entities, or commercial outfits.
“Tests are proliferating,” says Dr. Schmitz. “The more that are potentially out there, from a practical perspective, the better.”
A new saliva test for Covid-19
The newest test for the virus, one that uses saliva samples, was approved by the FDA on April 13. It is the first of its kind.
Certainly the test will be more comfortable for patients, says Dr. Seegmiller. It will also be safer for the person actually collecting the sample and means more people can be screened, according to a press release from Rutgers University, which developed the test.
“The impact of this approval is significant,” said Andrew Brooks, professor in the department of genetics at Rutgers in a prepared statement. “We can preserve precious personal protective equipment for use in patient care instead of testing. We can significantly increase the number of people tested each and every day.”
Right now, the tests are available to the RWJBarnabas Health network in New Jersey, according to the statement.

Antibody tests—looking for past infections
Antibodies are produced when the body mounts its immune response to an infection. Antibody tests can tell whether someone has been infected with Covid-19 in the past as opposed to having an active infection. With many other infections, having antibodies can mean you are immune to the virus, explains Dr. Ho. Unfortunately in the case of Covid-19, he adds, “there’s no direct proof that you’re protected. Every virus is different.”
The FDA approved a blood test for Covid-19 antibodies, from Cellex, in early April. Others have since been released and there are more on the way.
Despite these new tests, there are still a lot of questions about Covid-19 antibodies, just as there are questions about whether people can be reinfected with the virus.
“We don’t know if antibodies confer immunity,” says Dr. Seegmiller, echoing Dr. Ho. “The science is ongoing and so what it means that you have antibodies is not entirely clear.”
“We believe that having detectable antibodies is indicative of immunity to reinfection for some period of time, but we do not yet know how long that immunity may last, or how protective that immunity may be, adds Dr. Long. But antibody testing is just one piece of the puzzle when it comes to making public health decisions regarding Covid-19, he says.”It’s likely that the return to ‘normal’ life is going to be in phases, more gradual than how quickly distancing measures were implemented, and will very likely vary from city to city depending on the extent of local transmission in that area, and their hospital and testing capacity.”
This type of test can also be useful to see who may be eligible to donate their antibody-rich convalescent plasma to help people with Covid-19 in clinical trials, Dr. Long adds.
Home testing
On April 21, the FDA granted emergency clearance to a home test from a company called LabCorp, which plans to make the test available to frontline workers first and roll it out for general use in the following weeks (the test runs $119). The test only looks for an active infection—not antibodies or prior exposure.
Initially, Covid-19 home-testing kits were set to hit the market in late March when the FDA issued an alert warning that the tests were “unauthorized.” The companies making the kits—among them EverlyWell and Nurx—then postponed their launch, though EverlyWell has made its tests available to healthcare workers.
All the versions are at-home collecting tests, meaning you would receive a kit with everything you needed to do your own nasopharyngeal swab. You would then send the sample to a lab, which would do the actual testing. One concern experts had was how good the specimens would be, given that they weren’t obtained by a healthcare professional.

How Covid-19 tests have evolved
The CDC produced the first test for the Covid-19 virus, one that was then shipped out to labs in different states. Samples had to be sent back to the CDC to actually get tested.
The FDA then issued an Emergency Use Authorization (EUA) (on February 29) to allow labs throughout the country to start using tests they had designed. This bypassed the usual approval process for diagnostic tests. Vanderbilt University entered the game at about the same time, validating a test that was patterned after the CDC test and starting actual testing on March 9, four days after the first case of Covid-19 was reported in Tennessee, says Dr. Seegmiller.
The first day, Vanderbilt did six tests. On the 12th day, about 700 tests. Now they’ve evened out to about 200 or 300 a day.
Since then, individual institutions have developed tests based around the same CDC principles, says Dr. Schmitz, who is also the medical director of Vanderbilt’s Molecular Infectious Disease Laboratory. Now commercial entities are in the picture.
“As companies are ramping up production, we expect more of those will become available and will increase capacity and allow us to test more rapidly,” says Dr. Seegmiller.











