An RSV Vaccine Just Received FDA Approval for Older Adults
Updated: May 11, 2023
The new vaccine has been decades in the making.
Respiratory syncytial virus, or RSV, is a common respiratory virus that typically causes mild, cold-like symptoms. According to the Centers for Disease Control and Prevention (CDC), most people who catch RSV recover in a week or two; however, the virus can be severely dangerous for babies and seniors. It is the leading cause of bronchiolitis and pneumonia in infants 1 year old and younger. Each year, RSV leads to 80,000 hospitalizations of children under 5 and 177,000 hospitalizations of seniors.
Fortunately, help is on the way. On Wednesday, May 3, 2023, the Food and Drug Administration (FDA) officially approved the first vaccine against RSV. Here’s everything you need to know about this historic advancement in medicine.
What is the RSV vaccine?
The new vaccine is called Arexvy and was developed by the pharmaceutical company GSK, formerly known as GlaxoSmithKline. In GSK’s trials, Arexvy was found to be 82% effective at preventing lower respiratory illnesses caused by RSV. Furthermore, Arexvy was 94% effective for those who had at least one underlying medical condition.
Dr. Peter Marks, Director of the FDA’s Center for Biologics Evaluation and Research said in the FDA’s statement that the “approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States.”
Are there any risks to the RSV vaccine?
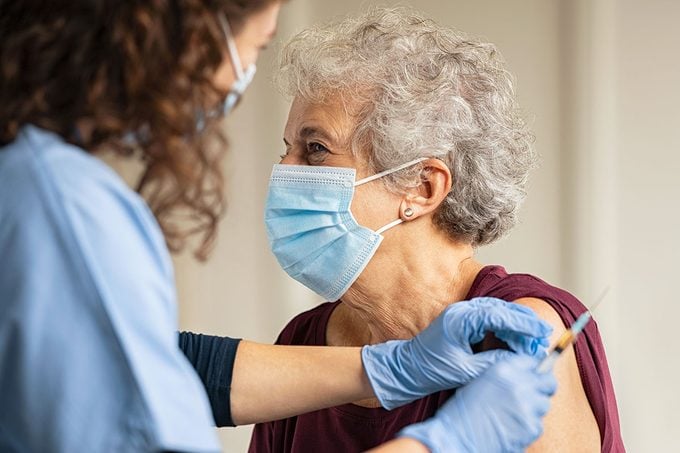
In February 2023, the FDA held an advisory panel meeting with GSK researchers. The researchers reported a few cases of neurological disorders in patients who had received the RSV vaccine. One 78-year-old woman in Japan was diagnosed with Guillain-Barré, a rare disorder in which the immune system attacks nerve cells about nine days after receiving the shot. Still, the FDA panel members decided to approve the vaccine, citing it as safe and effective.
Who is eligible for the RSV vaccine?
The RSV vaccine is specifically created and approved for administration in older adults. Back in March, the FDA recommended that adults aged 60 or older receive the vaccine. Arexvy will be administered to this vulnerable population as a shot.
What other RSV vaccines are in development?
There are several other RSV vaccines and immunizations under review currently. Three of the most notable include Sanofi’s monoclonal antibody immunization for infants (called nirsevimab), Pfizer’s RSV vaccine for people who are pregnant and Pfizer’s RSV vaccine for older adults, which has proven to be 85% effective at preventing lower respiratory tract illnesses caused by RSV. Hopefully, these vaccines will be further proven safe and approved, so that those people most vulnerable to RSV can receive appropriate protection soon.
Get The Healthy @Reader’s Digest newsletter and follow The Healthy on Facebook, Instagram, and Twitter. Keep reading:










