12 Biologics That Are Used to Treat Psoriasis
Updated: Jun. 17, 2021
Biologics have been game-changers for many people with psoriasis. Learn more about biologic medications used to treat psoriasis, including their potential risks and benefits.
Biologic therapy for psoriasis
In a relatively short period of time, biologic drugs have revolutionized the way doctors treat psoriasis and other autoimmune diseases.
There are now many biologics approved to treat psoriasis in the United States, with others in the pipeline.
The first-line treatment for psoriasis is often topical steroid creams, but if those don’t work, your doctor may prescribe a biologic medication right off the bat, says Bruce Strober, MD, PhD, clinical professor of dermatology at Yale University and founder of Central Connecticut Dermatology Research in Cromwell, Connecticut.
Doctors also turn to these drugs first when a large area of the body is affected by psoriasis or the psoriasis is taking a dramatic toll on quality of life.
“The impact of biologic therapy is indescribably enormous,” he says. “Medical dermatology is vastly different now than it was 20 years ago when I was in training.”
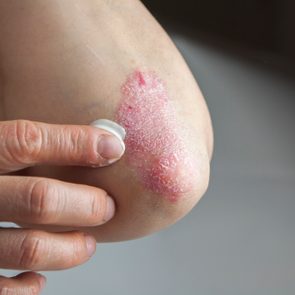
What is psoriasis?
Psoriasis is an inflammatory skin condition that affects 8 million Americans.
When you have psoriasis, your immune system kicks into high gear and revs up production of skin cells. Instead of shedding, these skin cells pile up on your skin, forming raised, reddened plaques that itch, burn, and are often covered with silvery scales.
Psoriasis can strike any part of your body, but it most commonly develops on the lower back, elbows, knees, legs, soles of the feet or palms, and/or scalp.
Psoriasis runs more than skin deep. One in three people with the condition may also develop psoriatic arthritis, which causes joint pain, inflammation, and limited mobility. This risk is even higher when psoriasis affects your scalp.
The underlying inflammation of psoriasis is also linked to a greater risk for heart disease and diabetes. What’s more, psoriasis can affect your self-esteem and self-confidence, particularly if it remains untreated or inadequately treated.
What are biologic drugs?
Given as IV infusions or injections at home or in a doctor’s office, biologic drugs are genetically engineered proteins that target specific parts of the immune system that drive inflammation.
There are several classes of biologic drugs; they are organized largely by which inflammatory protein or proteins they target.
Categories of biologics include tumor necrosis factor inhibitors (TNF inhibitors), interleukin inhibitors, B-cell inhibitors, and selective costimulation modulators (immunomodulators). TNF blockers and interleukin inhibitors are the classes of biologics currently used to treat psoriasis, though there’s an immunomodulator that can treat psoriatic arthritis.
Biologics can be used with other psoriasis treatments, including UV light therapy, topical steroids, or systemic medications such as methotrexate. Or they might be used on their own.
Biologics versus older psoriasis medications
Biologics target specific parts of the immune system that cause inflammation.
By contrast, non-biologic drugs that treat psoriasis—like methotrexate or cyclosporine—take a broader approach and suppress the entire immune system, explains Sam Hwang, MD, PhD, chair and professor of dermatology at the University of California Davis.
Think of the immune system as a line of dominoes. The older drugs take no prisoners, knocking down the entire line. Biologics, on the other hand, start midway through the lineup, blocking inflammation only from that point on.
This is why biologics tend to cause fewer side effects than older drugs, Dr. Hwang explains.
Biologics have been game-changers for many people with psoriasis. It’s now possible to achieve clear skin—with no plaques—in weeks. This is much faster than what has been seen with older disease-modifying psoriasis drugs, he says.
They also have a convenience edge.
“You can give yourself injections at home in the fat layer under your skin, similar to insulin injections for people with diabetes,” Dr. Hwang says.
But unlike insulin, these shots aren’t given daily. “Some are only given every 12 weeks,” he says. “This is extremely convenient.”
Your doctor will have to teach you or your caregiver how to self-inject these medications before giving you the green light to do so at home.

Biologics for psoriasis
There are several biologics that work to treat psoriasis, and which one will be best for you depends on a number of factors. You’ll work with your doctor to discuss options, weighing the pros and cons of the drugs below.
TNF inhibitors
Tumor necrosis factor is an inflammatory protein that is seen in abundance in psoriasis and psoriatic arthritis. Blocking this protein stops the inflammation cascade from continuing.
Drugs in the TNF blocker class that treat psoriasis include:
Cimzia (certolizumab pegol)
Approved for adults with moderate to severe psoriasis and/or psoriatic arthritis, Cimzia is given as an injection under the skin. After an initial dose, you will need to inject yourself every other week to treat psoriasis.
This drug also has approval from the U.S. Food and Drug Administration (FDA) for other autoimmune conditions, including Crohn’s disease, rheumatoid arthritis, and a type of inflammatory back pain called ankylosing spondylitis.
Enbrel (etanercept)
Approved for people with moderate to severe psoriasis and/or psoriatic arthritis, Enbrel is given twice weekly by self-injection under the skin for three months. Then you will need to inject yourself with a maintenance dose once a week to keep psoriasis symptoms at bay.
It can be given weekly in kids with moderate to severe psoriasis. It is also approved for ankylosing spondylitis, rheumatoid arthritis, and juvenile idiopathic arthritis.
Humira (adalimumab)
Humira is approved to treat moderate to severe psoriasis and psoriatic arthritis as well as other autoimmune diseases, such as Crohn’s disease and rheumatoid arthritis.
Given as a self-injection, Humira is administered every other week, starting one week after the initial dose.
Remicade (infliximab)
This TNF blocker is approved for psoriatic arthritis and chronic severe psoriasis as well as other autoimmune diseases.
It’s given in a doctor’s office by intravenous infusion as an initial dose, followed by additional infusions two and six weeks later. After that, infusions occur every eight weeks.
Other TNF blockers—namely, Simponi (golimumab) and Simponi Aria (golimumab)—are approved for psoriatic arthritis, the National Psoriasis Foundation notes.
Interleukin 17 (IL-17) inhibitors
Drugs in this class of biologics block IL-17, another inflammatory protein that plays a role in psoriasis and psoriatic arthritis.
Cosentyx (secukinumab)
The IL-17 blocker Cosentyx is given to people with psoriasis, initially by self-injection under the skin and then once weekly for four weeks. After that, it’s given every four weeks.
This drug also has an FDA nod for moderate to severe scalp psoriasis, psoriasis in kids, and other autoimmune diseases.
Siliq (brodalumab)
Siliq is an IL-17 blocker that is approved to treat moderate to severe plaque psoriasis in adults. It can be given as a self-injection under the skin initially and then once a week for two weeks. Following that lead-up period, injections are given every two weeks.
Taltz (ixekizumab)
Taltz is approved for the treatment of moderate to severe plaque psoriasis in people ages 6 and older. It is given via self-injection under the skin.
For adults, the initial dose is given during the first week of treatment and again every two weeks until week 12, then every four weeks thereafter.
In kids, the dosing schedule is different. After an initial dose, kids are treated every four weeks.
It’s also approved for psoriatic arthritis, ankylosing spondylitis, and non-radiographic axial spondyloarthritis, another form of spinal arthritis.
Interleukin 23 (IL-23) blockers
IL-23 is another protein involved in the inflammation cascade of psoriasis.
Ilumya (tildrakizumab-asmn)
The IL-23 blocker Ilumya is approved for psoriasis. It is given by injection under the skin initially and then four weeks later. Maintenance injections are given every 12 weeks.
Skyrizi (risankizumab-rzaa)
Skyrizi has the FDA’s approval to treat moderate to severe plaque psoriasis in adults. You give yourself an injection under the skin the first week of treatment, in week four, and every 12 weeks thereafter.
Tremfya (guselkumab)
Tremfya is an IL-23 blocker that’s approved for moderate to severe plaque psoriasis in adults as well as adults with active psoriatic arthritis.
It’s given via self-injection under the skin at the start, four weeks later, and then every eight weeks after that.
Interleukin 12 and 23 (IL-12 and IL-23) inhibitor
Stelara (ustekinumab) selectively targets IL-12 and IL-23, both of which are involved in the inflammation cascade that leads to psoriasis.
It is given by self-injection under the skin initially, again at week four, and then every 12 weeks thereafter. It is also approved to treat psoriatic arthritis, Crohn’s Disease, and ulcerative colitis.
Selective costimulation modulator
While most biologic drugs that treat psoriasis fall into the TNF blocker or interleukin inhibitor classes of medication, there’s an immunomodulator called Orencia (abatacept) that is approved for psoriatic arthritis.
This T-cell inhibitor is delivered montly via IV infusion at a doctor’s office or weekly via self-injection at home.
It’s also approved for rheumatoid arthritis and polyarticular juvenile idiopathic arthritis.
Biosimilars for psoriasis
Biosimilars are essentially generic versions of biologic drugs. They are more like twin siblings than cousins of the originator drug.
For approval, the FDA must determine that the biosimilar is highly similar to the original and has no clinically meaningful differences.
Biosimilars will likely be less costly than their originating biologics, Dr. Hwang says.
The list of approved biosimilars includes:
Biosimilars for adalimumab
- Amjevita (adalimumab-atto)
- Abrilada (adalimumab-afzb)
- Cyltezo (adalimumab-adbm)
- Hadlima (adalimumab-bwwd)
- Hulio (adalimumab-fkjp)
- Hyrimoz (adalimumab-adaz)
Biosimilars for etanercept
- Erelzi (etanercept-szzs)
- Eticovo (etanercept-ykro)
Biosimilars for infliximab
- Avsola (infliximab-axxq)
- Inflectra (infliximab-dyyb)
- Ixifi (infliximab-qbtx)
- Renflexis (infliximab-abda)
If biosimilars are less expensive, insurance companies may be more likely to foot the bill, which should mean more people with psoriasis will have access to these quality-of-life-saving medications, Dr. Hwang points out.
“When available, if they can lower the cost of treating patients, physicians and patients will both welcome biosimilars,” he says.
As of now, infliximab-axxq (Avsola), infliximab-dyyb (Inflectra), and infliximab-abda (Renflexis) are available, but others are stalled by legal delays, according to the Arthritis Foundation.
Biologics for psoriasis: side effects and concerns
Like other medications, biologics and biosimilars do have risks and side effects.
These may include:
- Upper respiratory infections
- Stomach pain
- Flu-like symptoms
- Headache
- Injection-site reactions
Infections are the main risk, Dr. Hwang says.
“Your doctor will rule out tuberculosis before starting you on a biologic, and then test again once a year,” he says.
There’s a lot less monitoring involved with biologics compared with older drugs because of the lower risk of potential side effects.
“A battery of lab tests is required with older immune-system-suppressing drugs for psoriasis, like cyclosporine and methotrexate, but not biologics,” says Dr. Hwang. “I see some of my patients on biologics every six months.”
Decisions, decisions
With so many biologics available to treat psoriasis, how does your doctor decide?
The discussion takes several factors into account, including what type of psoriasis you have, any other medical conditions, your preferences, as well as cost.
Considering safety
Some conditions may make a biologic drug unsafe, including a history of cancer, heart failure, liver diseases, and/or multiple sclerosis.
Some biologics, such as TNF blockers have been around longer than others, so they are often first in line, Dr. Hwang says.
“We tend to start with anti-TNF drugs, and then if they are not working, we will move to other classes or newer agents, such as IL-17 or IL-23 blockers, which may be more expensive,” he says.
There is a caveat: TNF blockers may cause new or worsening heart failure. “If a patient has heart failure or is at risk for it, we probably won’t pick an anti-TNF drug, as other biologics don’t have these contraindications,” Dr. Hwang says.
Considering other conditions
If you also have psoriatic arthritis, your doctor will likely start with a biologic that also has FDA approval for psoriatic arthritis, Dr. Hwang says.
Trial and error
There are no official guidelines or tests that can say a certain person with a certain type of psoriasis can benefit from a certain biologic … yet.
“It’s really hard to predict which one is guaranteed to work and what side effects may occur,” says Dr. Hwang.
Biologics need to be taken for the long haul. Stopping can bring on a return of symptoms. If one biologic doesn’t work for you, your doctor can try another, and another still. There is typically some trial and error involved in finding the right biologic.
There’s also a risk of becoming resistant to the effects of one biologic.
“Your body may develop anti-drug antibodies that will make it less likely to work,” Dr. Hwang says. “We see drug failure after two to five years and then switch to another drug.”
This occurs more frequently if you stop and start a biologic, he adds.
Barriers to widespread use
When biologics first came on the scene, guidelines called for starting with older and less-expensive disease-modifying drugs, such as methotrexate and cyclosporine, but now doctors are increasingly starting biologics earlier when and where they can.
The main impediment? Cost.
Access to biologics has been impeded by the high costs of these drugs. Some insurers require that you try—and fail—other therapies before starting on a biologic for your psoriasis. This is known as step therapy, and it is meant to rein in costs.
“Access is not bad,” says Dr. Strober. “However, the prior authorization work needed to deliver the drugs and imposed on a busy practice is onerous.”
Biologics for psoriasis and Covid-19
The U.S. Centers for Disease Control and Prevention (CDC) has relaxed some of its requirements about face coverings for fully vaccinated people.
But if you have psoriasis and are taking medications that affect the immune system, including some biologics, you should continue wearing a mask and social-distancing when you are in contact with unvaccinated people or those whose vaccination status is unknown, the National Psoriasis Foundation states.
Make sure to ask your doctor for guidance.